Galvanic Corrosion 4
Copper and its alloys are widely used as piping and heat exchanger tubes to handle waters with a wide range of chloride contents, from drinking water to seawater. Sometimes there is a leak due to some form of localised corrosion and a small piece of new pipe, or a few new tubes are installed to enable use to continue.
Sometimes the new piece of pipe or tube fails very rapidly, much to everyone's surprise. The reason for this is that, in some waters, there can be a significant potential difference (30 to 70mV) between new and filmed metal. The potential difference depends on the composition and temperature of the water. If the area of the old (filmed) metal is very much greater than that of the new metal, accelerated corrosion will occur until the new metal forms a protective film. Sometimes the corrosion is so rapid that perforation occurs before a protective film forms.
This is usually seen with copper and its alloys because they efficient cathodes, even when filmed, and can stimulate anodic dissolution readily. This does not occur with stainless steels and other metals that form passive films.
Posted on: 7th April 2017
Alloys 400/K-500
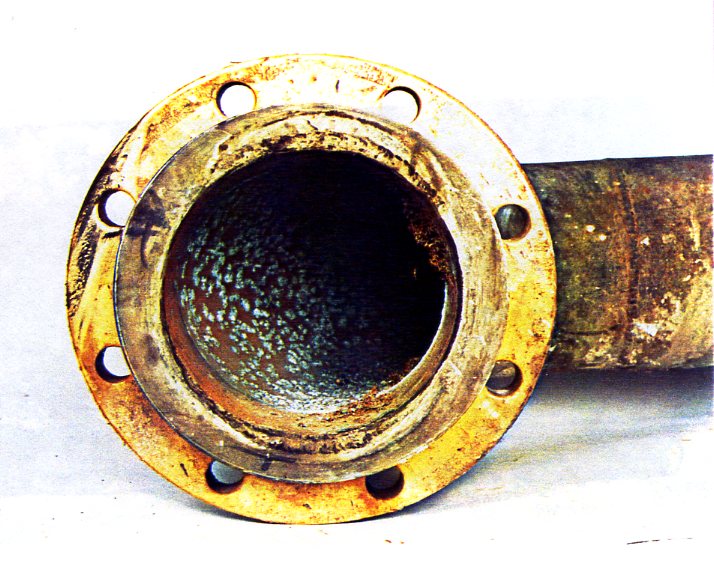
These materials (UNS N04400 and N05500) are nickel-copper alloys, with the K-500 grade being capable of having increased strength by precipitation hardening. The alloys were invented in the 1950's and have been widely used in seawater. Although they have good resistance to flowing seawater, they are susceptible to pitting and crevice corrosion, particularly in slow flowing natural seawater (as opposed to chlorinated seawater). The picture below shows a badly pitted alloy 400 seawater pipe from a Shell plant. Alloy K-500 has often been used for high strength bolting, but it can suffer crevice corrosion in seawater unless it is galvanically protected.
Alloys 400 and K-500 are not galvanically compatible with high alloy stainless steels and Ni-Cr-Mo alloys (e.g. alloy 625) in seawater, and will suffer accelerated atttack if coupled.
Because these alloys have a high nickel content, there are more cost-effective modern alloys that can meet many of the properties of alloys 400 and K-500. To replace them with copper alloys, it is coomon to select C70600 or C71500 instead of alloy 400, and C72420 as an alternative to K-500. Alloy 400 may also be replaced with cast or wrought superduplex stainless steel, while alloy K-500 may be replaced with cold worked superduplex or nickel alloys N07716 and N07725.
Posted on: 21st March 2017
Leaded Copper Alloys
These include leaded brasses, such as UNS C3800, manganese bronze and the leaded nickel silvers. The lead is usually present at 1 to 2 wt.% to improve machineability. The lead particles are fine and are distributed more or less uniformly throughout the matrix.
Sometimes these alloys are used for components that operate above the melting point of lead (~328°C). The author has seen these alloys used for items such as gas jets in a baker's oven and steam glands in a power station. At temperatures greater than 328°C the lead is very mobile and over a period of weeks to months it diffuses through the matrix and forms a small number of large lead particles. Where these are at the exposed surface, the liquid lead encourages fast diffusion and rapid corrosion can occur if air is present.
The solution is to use lead-free copper alloys or a chromium steel, such as the 7% or 9%Cr grades.
Posted on: 7th March 2017
Galvanized Steel
Galvanized steel piping is widely used to handle water, particularly fresh waters. It relies for its corrosion resistance on the formation of protective films of zinc and calcium carbonate. This means that medium and hard waters, with a total alkalinity>100mg/L (as CaCO3) and a positive Langelier Index will form protective scales. However, soft and particularly soft, acidic waters will not be able to form scale and they will corrode more rapidly. However, the low conductivity and low dissolved solids content of such waters means that the corrosion rate is usually low enough to be acceptable (<0.1mm/y).
In seawater galvanized steel offers no advantages over bare carbon steel. The reason for this is that, despite the total alkalinity of seawater exceeding 100mg/L, the high chloride content means that zinc corrosion products are soluble at normal seawater pH (8.2). Hence, the zinc layer dissolves rapidly and then the pipe corrodes just like carbon steel.
Posted on: 21st Feb 2017
Martensitic Stainless Steels
These are alloys that can be heat treated to give high proof stresses and tensile strengths and they are widely used in industry where high strength and some corrosion resistance is required. Such alloys include UNS S41000, S42000 and precipitation hardening grades such as J91540 and S17400. These alloys have chromium contents from about 12 to 17% plus small additions of nickel and other elements.
However, the corrosion resistance of these alloys is not very good in oxidizing media, and is inferior to that of the 300 series austenitics ,e.g. 304L and 316L, because of their lower chromium content. While they have some resistance to atmospheric corrosion, they have little resistance to corrosion in oxidizing, aqueous media, e.g. fresh water.
Where high strength is required and corrosion resistance in an oxidizing medium, then it is safer to use a duplex stainless steel or a heat treatable nickel alloy, with good corrosion resistance in the medium of interest.
Where the martensitic stainless steels have good corrosion resistance is in reducing media, such as sweet and sour oil and gas fluids. Even in these media, there can be a risk of sulphide SCC and adherence to correct heat treatment and hardness requirements is essential, as specified in ISO15156/ NACE MR01075 (upstream) and NACE MR0103 (downstream).
Posted on: 7th Feb 2017

