Galvanic Corrosion 3
Steel, galvanized steel and copper pipes and fittings are all frequently used to handle fresh waters. Copper is not galvanically compatible with steel or galvanized steel and measures areusually taken to avoid using them in contact. However, even when they are not in contact, accelerated corrosion of steel and galvanised steel can still occur.
If copper, or its alloys, are used upstream of steel or galvanised steel, then corrosion can still occur if copper corrosion products are carried downstream and settle on the steel/galvanized steel. This is particularly prevalent in warm and hot waters. This corrosion mechanism has caused a numberof expensive failures in blocks of flats with a common hot water system. For this reason it is usual to specify that copper and copper alloys may only be used downstream of steel and galvanized steel.
Posted on: 21st Jan 2017
Erosion Corrosion
Copper pipes are widely used for handling fresh and drinking water and generally they perform well and last for many years. However, occasionally there are leaks, and one cause of failure is erosion corrosion. This is caused when the water flow is so turbulent that it destroys the protective film on the metal surface, which then corrodes and forms the film again, which is again destroyed, and so on.
There are recommendations for safe velocities with copper tubes in fresh waters, but these can depend on water temperature. A UK recommendation is a maximum velocity of 1.0m/s in hot water (50 to 60°C), which can be increased to 2.0m/s in cold water (10 – 20°C. However, the turbulence can be greatly increased after sharp bends, and if these are present it is prudent to reduce the flow velocity. The alternative is to use large radius bends to minimise the turbulence.
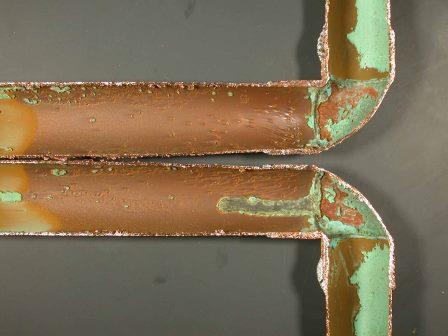
Erosion corrosion of copper pipe after sharp bend.
Posted on: 7th Jan 2017
Sigma Phase
There is always a concern about duplex stainless steels and the presence of sigma phase. This is an intermetallic phase that can precipitate due to poor heat treatment or to excessive heat inputs during welding. Sigma phase causes a loss in both toughness and corrosion resistance.
Provided care is taken during manufacture and fabrication, sigma phase should not be a problem. However, when it occurs, the question is often asked, how much is acceptable. There is not a simple answer to this question, because the effect of sigma phase depends on its size. The larger the diameter of the sigma particle, the greater is the area around it that is denuded in chromium and molybdenum, and generally, the more detrimental is its effect on properties. However, a wide range of particle sizes is possible and it is better to conduct fitness for purpose tests if sigma is suspected.
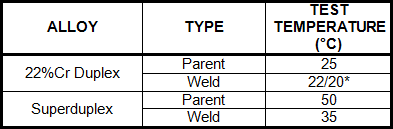
The most common tests are a Charpy impact toughness test at the desired temperature (often -46 or -50°C) and an ASTM G48 method A corrosion test. The minimum Charpy energy has been suggested as 70J for parent metal, as it will decrease further on welding, and 35J for welds. In the corrosion test, the following temperatures have been recommended, with the pass criteria being no pitting and a weight loss < 4g/m2.
Recommended ASTM G48 test temperatures for duplex stainless steels.
Posted on: 21st Dec 2016
Start-Up
In seawater cooling systems a leak of the tubing or piping costs money and loses production. However, it has been demonstrated that the use of a “soft” start-up is of great benefit to many alloys and reduces the risk of a failure.
Copper alloys are widely used for heat exchangers and seawater piping, but in the presence of pollutants, such as sulphide and ammonia, they can suffer from accelerated localised corrosion, leading to failure. Once protective films form in clean, natural seawater, they are much more resistant to corrosion by pollutants. As pollution is often intermittent, a long life can be achieved by a start-up when no pollution is present. Simple monitoring of the seawater at the inlet can determine the presence of both sulphide and ammonia.
High alloy stainless steels, such as the 6% Mo austenitic and 25% Cr superduplex alloys, are limited by the maximum temperature of seawater that they can tolerate without suffering crevice corrosion or pitting at welds. This is typically 30 to 40°C, depending on the alloy. However, where discharge temperatures from heat exchangers are higher, it has been shown that running the system with cooler water first, strengthens the passive film and enables operation at 10 to 15°C higher than normal. One recommended start up regime is:
2 days minimum with cold, untreated seawater
5 days minimum with cold chlorinated seawater
Thereafter, turn on heat exchangers.
A superduplex seawater piping system was given a soft start-up and the piping after the heat exchangers ran at 55°C without any problems.
Posted on: 7th Dec 2016
Change of Use
It is quite common to re-use components from one system in a new one, to save money. However, this may cause premature failure unless attention is paid to the conditions of use, compared with the previous service.
The photograph below shows an aluminium alloy shell, naval brass tube plates and aluminium brass tubes from a cooler that originally had seawater as the coolant in the tubes and lubricating oil on the shell side. Then the unit was used as a water heater with brackish water on both sides of the unit. The aluminium alloy shell suffered rapid galvanic corrosion coupled to the large area of copper alloy and leaked very quickly. The use of an aluminium alloy shell with copper alloy tubes is only acceptable with a non-conducting process-side fluid, such as lubricating oil.

Corrosion of aluminium alloy shell in a copper alloy heat exchanger.
Posted on: 21st Nov 2016

