Rapid Tests for Anions and Cations
When trying to determine the cause of corrosion it is common to examine a sample in the SEM and analyse the corrosion products. However, this only detects elements and cannot tell if the sulphur is present as sulphide or sulphate, for example.
There is a quick and simple way to test for many species using spot tests. All of the ones below were taken from "Spot Tests in Inorganic Analysis" by F Fiegl, published by Elsevier, 2nd edition, 1958. There are probably more recent editions. All you need to do is to scrape a small sample of corrosion product onto a glass slide, add a drop or two of the test solution and observe under a low power microscope (X10 or X20).
SULPHIDE - This is readily detected with the sodium azide test and it is not only specific for sulphide, it is also very sensitive. The rate at which nitrogen bubbles evolve in the solution gives an indication of the quantity of sulphide, from a trace to a large quantity.
CHLORIDE - If you suspect chloride has entered a system where it was not expected, the silver nitrate test is also very sensitive to chloride and the amount of white precipitate indicates how much chloride is present.
AMMONIA- this is a more fiddly test and must be done very quickly after removal as ammonia is so volatile. However, I have used this to demostrate ammonia-induced crevice corrosion in copper-nickel heat exchanger tubes.
There are lots of tests for other anions and cations and many are very specific and they are all quick to do. Make sure you test the solution on something of known composition before using, as some of the test solutions can go off, when exposed to daylight, or just with time.
Posted on: 21st May 2017
Sulphur and Sulphides
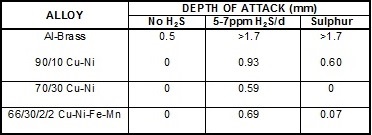
It is well known that the corrosion of copper alloys in aerated seawater is greatly accelerated when small amounts of sulphide (eg cysteine or hydrogen sulphide) are also present. As little as 0.02 mg/L sulphide can greatly increase corrosion and cause rapid failure of heat exchanger tubes.
However, if the seawater feed lines are long (> 100m) the dissolved oxygen in the seawater reacts with the sulphide to form elemental sulphur. This is less aggressive to copper alloys compared with sulphide although the reduction in severity varies with alloy, as shown in the table below. This data was generated at BNF Metals Research Association in the 1950s but was never published in the open literature.
Depth of Impingement Attack in Seawater using the BNF Jet Test at 5m/s and 20°C
Posted on: 7th May 2017
Etching of Duplex Stainless Steels
Modern duplex stainless steels have a phase balance around 50/50 austenite/ ferrite and many specifications require the ferrite content to be determined. A common requirement is to etch electrolytically in 40% NaOH or KOH, because this stains the ferrite light brown and makes ferrite determination easier. However, this etch does not clearly show grain boundaries or third phases, such as sigma, chi and nitrides. These are best shown by first etching in 10% oxalic acide followed by the hydroxide stain etch (see below). If the microstructure is viewed by polarised light or bright field illumination, the sigma/chi phases appear orange. Nitrides appear as black dots in the ferrite or decorate the ferrite sub-grain boundaries (see below).
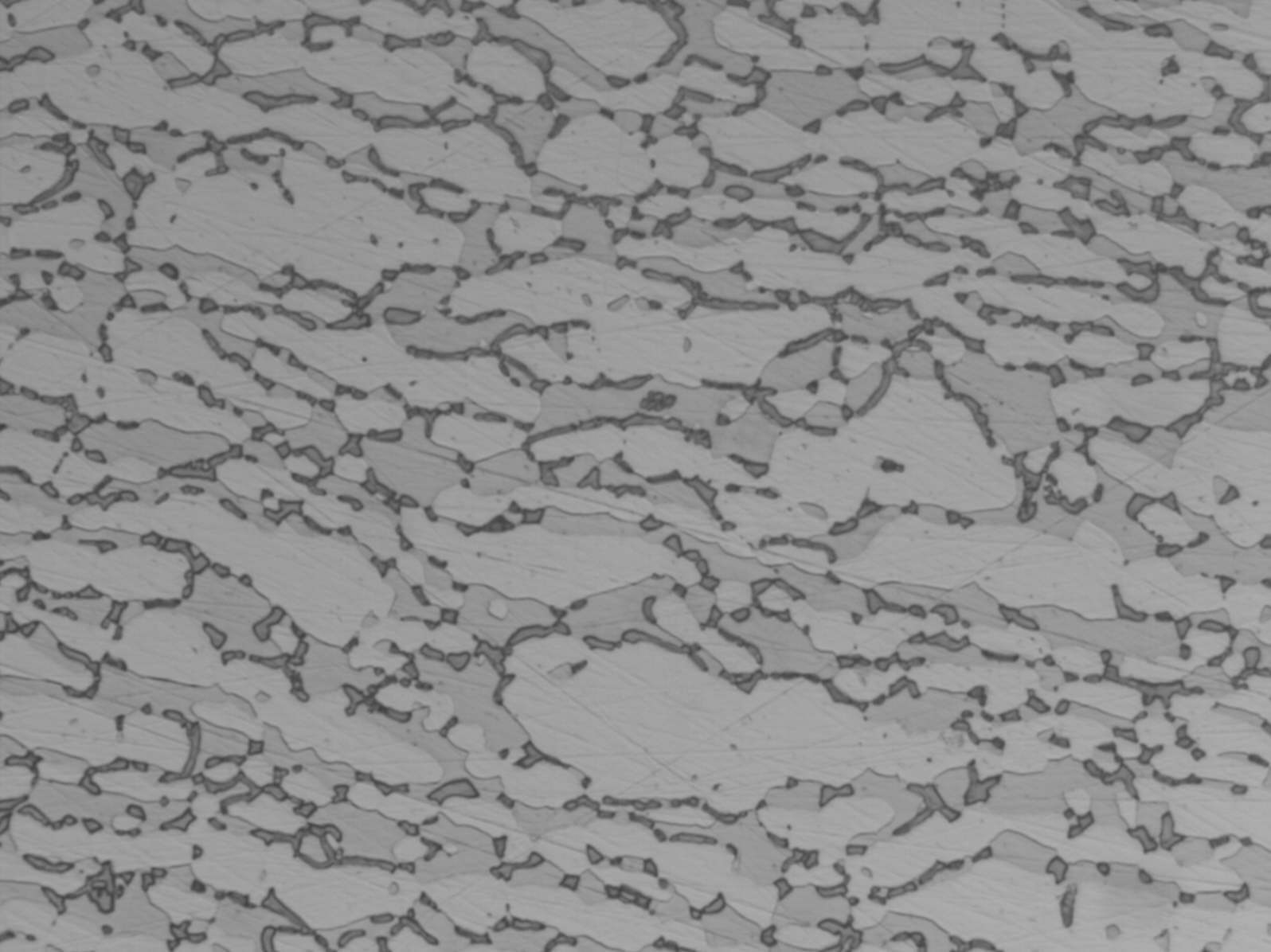
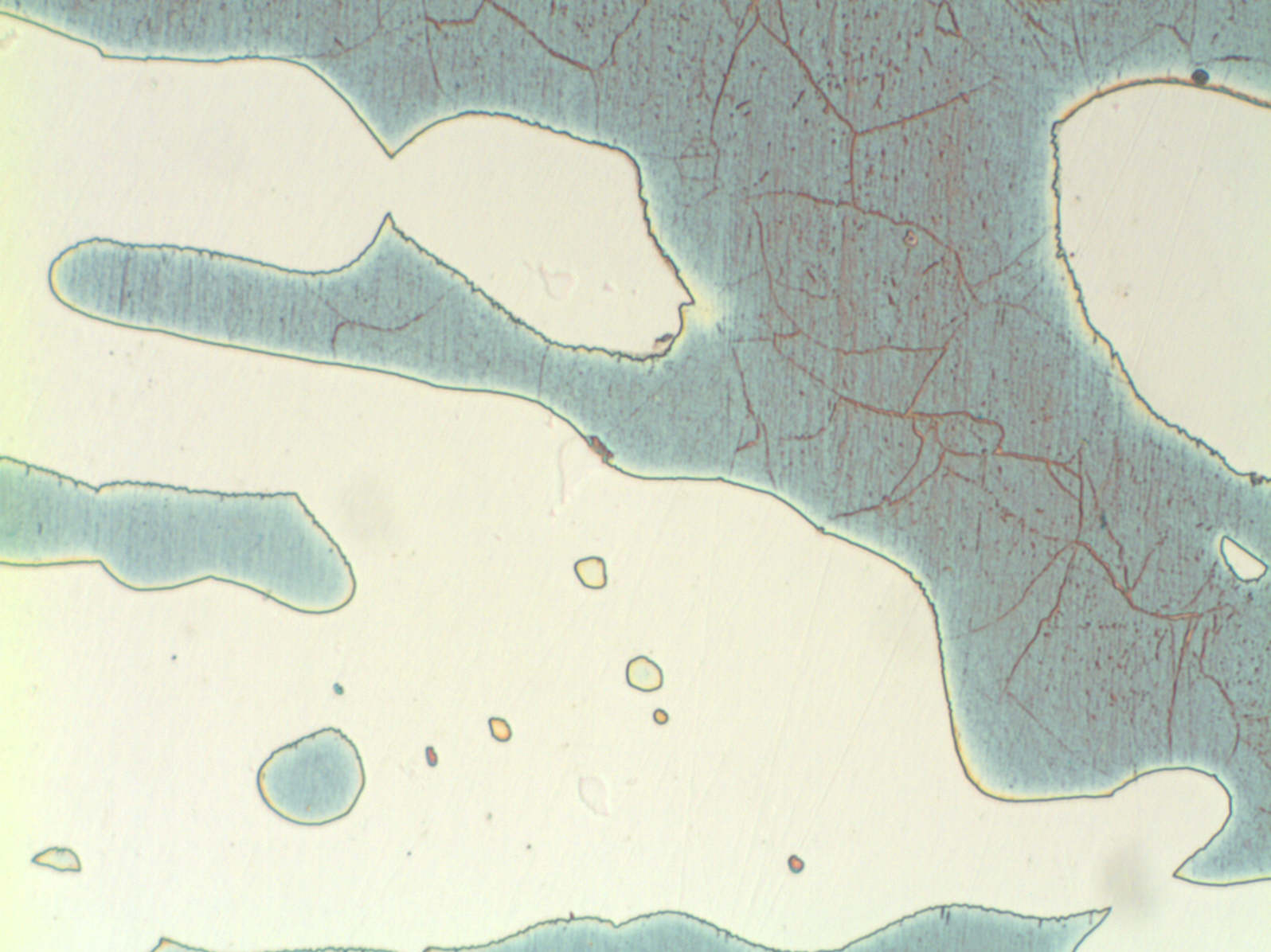
UNS S32760 showing sigma and chi phases UNS S32760 showing nitrides in the ferrite
Posted on: 21st April 2017
Galvanic Corrosion 4
Copper and its alloys are widely used as piping and heat exchanger tubes to handle waters with a wide range of chloride contents, from drinking water to seawater. Sometimes there is a leak due to some form of localised corrosion and a small piece of new pipe, or a few new tubes are installed to enable use to continue.
Sometimes the new piece of pipe or tube fails very rapidly, much to everyone's surprise. The reason for this is that, in some waters, there can be a significant potential difference (30 to 70mV) between new and filmed metal. The potential difference depends on the composition and temperature of the water. If the area of the old (filmed) metal is very much greater than that of the new metal, accelerated corrosion will occur until the new metal forms a protective film. Sometimes the corrosion is so rapid that perforation occurs before a protective film forms.
This is usually seen with copper and its alloys because they efficient cathodes, even when filmed, and can stimulate anodic dissolution readily. This does not occur with stainless steels and other metals that form passive films.
Posted on: 7th April 2017
Alloys 400/K-500
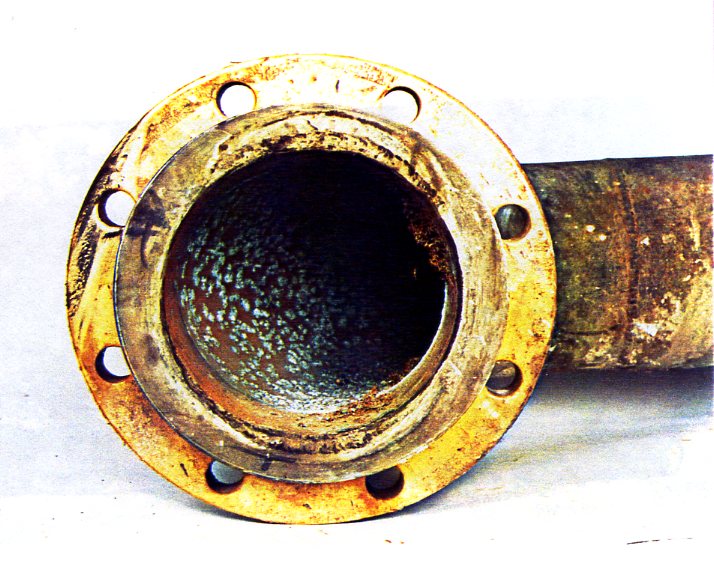
These materials (UNS N04400 and N05500) are nickel-copper alloys, with the K-500 grade being capable of having increased strength by precipitation hardening. The alloys were invented in the 1950's and have been widely used in seawater. Although they have good resistance to flowing seawater, they are susceptible to pitting and crevice corrosion, particularly in slow flowing natural seawater (as opposed to chlorinated seawater). The picture below shows a badly pitted alloy 400 seawater pipe from a Shell plant. Alloy K-500 has often been used for high strength bolting, but it can suffer crevice corrosion in seawater unless it is galvanically protected.
Alloys 400 and K-500 are not galvanically compatible with high alloy stainless steels and Ni-Cr-Mo alloys (e.g. alloy 625) in seawater, and will suffer accelerated atttack if coupled.
Because these alloys have a high nickel content, there are more cost-effective modern alloys that can meet many of the properties of alloys 400 and K-500. To replace them with copper alloys, it is coomon to select C70600 or C71500 instead of alloy 400, and C72420 as an alternative to K-500. Alloy 400 may also be replaced with cast or wrought superduplex stainless steel, while alloy K-500 may be replaced with cold worked superduplex or nickel alloys N07716 and N07725.
Posted on: 21st March 2017

