Galvanic Corrosion 5
Galvanic corrosion in fresh waters is somewhat different to that in seawater or strong brines. The potentials of common alloys are a little different to those in seawater, but the relative ranking is more or less the same. However, the lower conductivity of the water (~100X) means that galvanic corrosion is often confined close to the metal junction. In addition, the low conductivity can mean that current from a remote cathode is greatly reduced. However, this is also affected by geometry. Stainless steel is cathodic to copper in fresh waters, but stainless steel pipes have been successfully been joined with copper solder fittings. Where the area ratio of stainless steel to copper is large, severe galvanic corrosion can occur. The photograph below shows a badly corroded copper pipe entering a large stainless steel hot water tank.
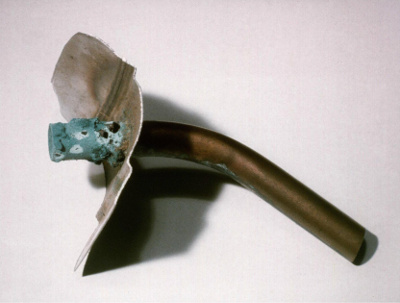
Badly corroded copper pipe entering a large stainless steel hot water tank.
Posted on: 21st August 2017

