Etching of Duplex Stainless Steels
Modern duplex stainless steels have a phase balance around 50/50 austenite/ ferrite and many specifications require the ferrite content to be determined. A common requirement is to etch electrolytically in 40% NaOH or KOH, because this stains the ferrite light brown and makes ferrite determination easier. However, this etch does not clearly show grain boundaries or third phases, such as sigma, chi and nitrides. These are best shown by first etching in 10% oxalic acide followed by the hydroxide stain etch (see below). If the microstructure is viewed by polarised light or bright field illumination, the sigma/chi phases appear orange. Nitrides appear as black dots in the ferrite or decorate the ferrite sub-grain boundaries (see below).
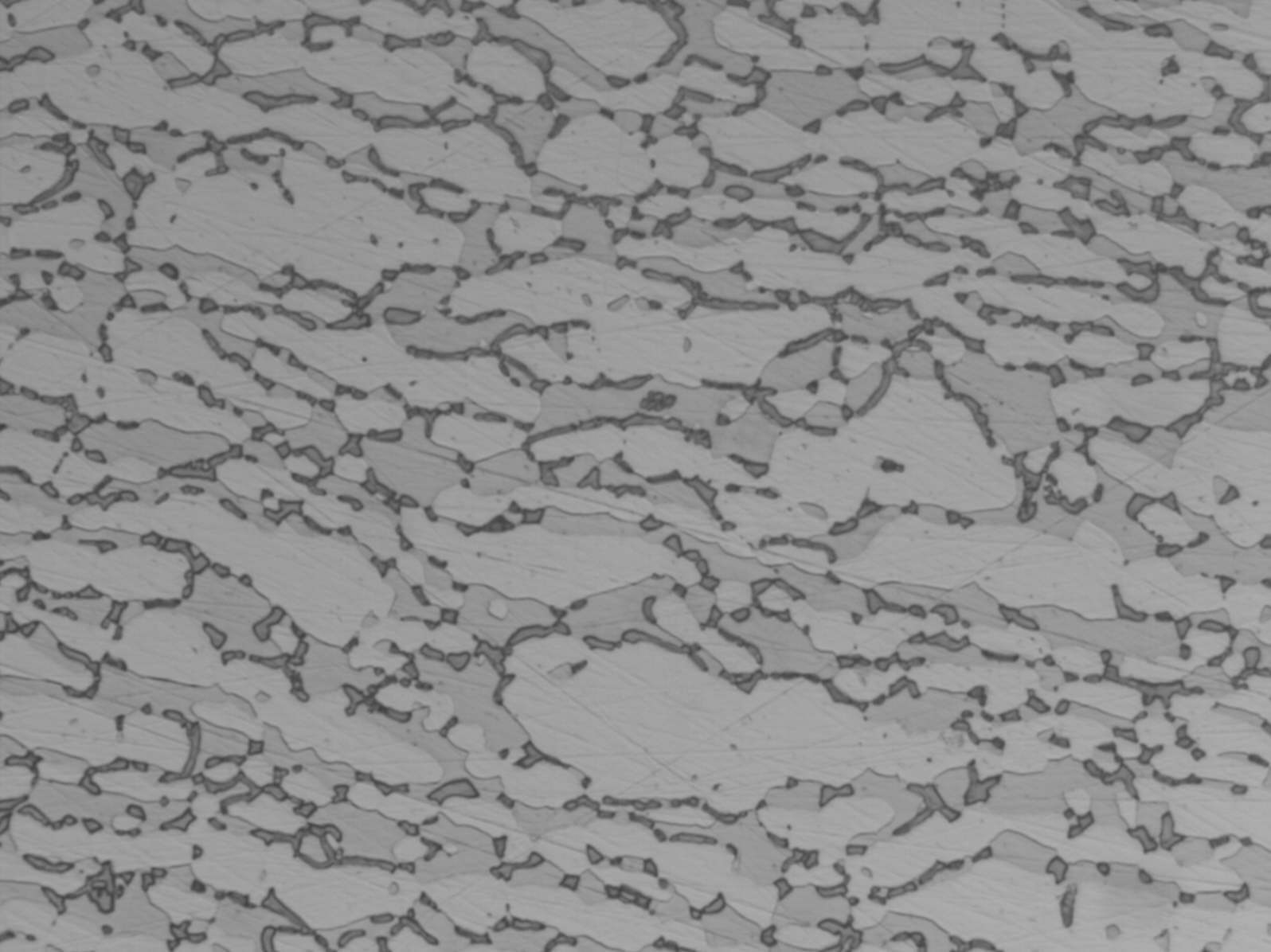
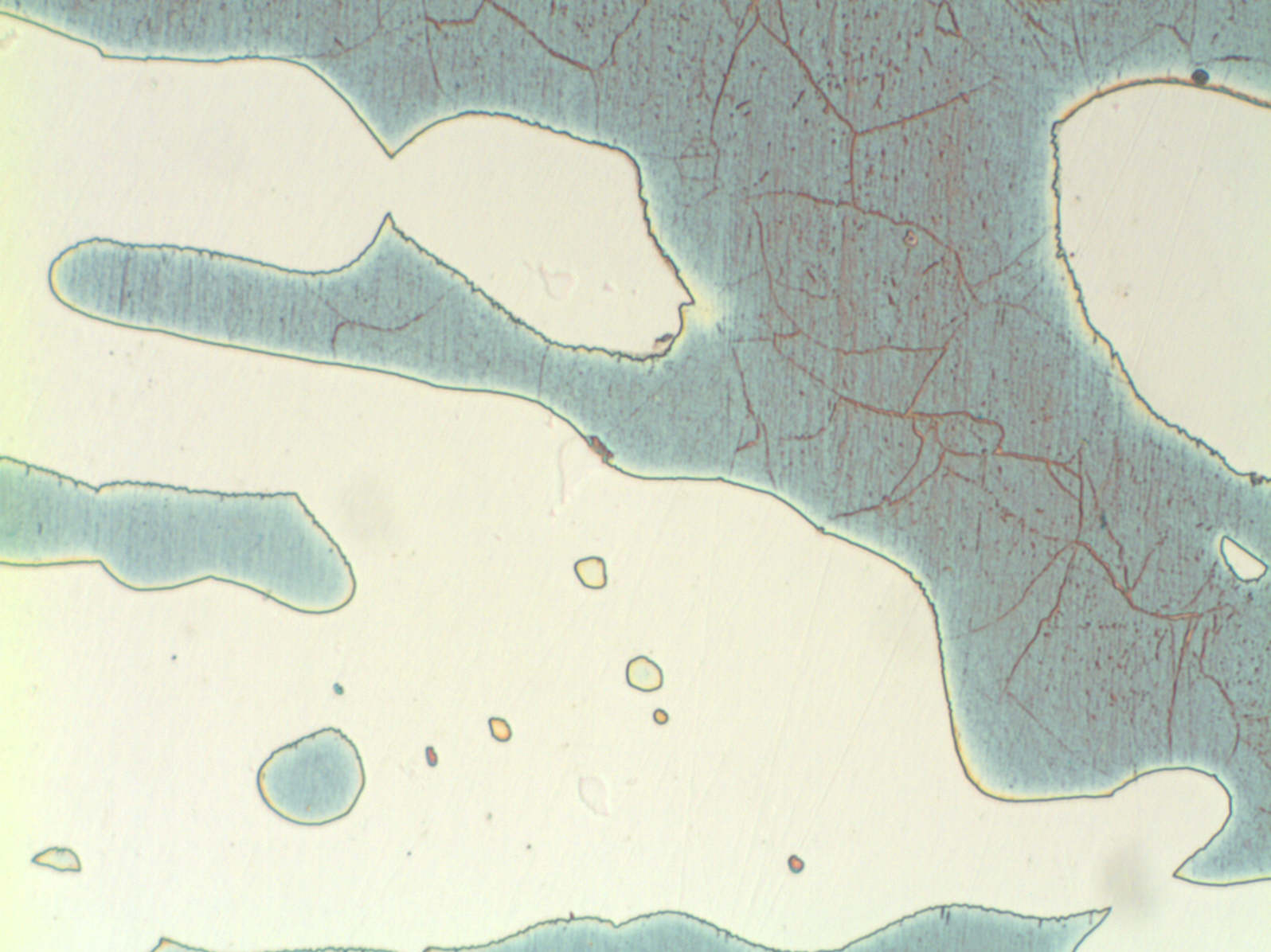
UNS S32760 showing sigma and chi phases UNS S32760 showing nitrides in the ferrite
Posted on: 21st April 2017
Galvanic Corrosion 4
Copper and its alloys are widely used as piping and heat exchanger tubes to handle waters with a wide range of chloride contents, from drinking water to seawater. Sometimes there is a leak due to some form of localised corrosion and a small piece of new pipe, or a few new tubes are installed to enable use to continue.
Sometimes the new piece of pipe or tube fails very rapidly, much to everyone's surprise. The reason for this is that, in some waters, there can be a significant potential difference (30 to 70mV) between new and filmed metal. The potential difference depends on the composition and temperature of the water. If the area of the old (filmed) metal is very much greater than that of the new metal, accelerated corrosion will occur until the new metal forms a protective film. Sometimes the corrosion is so rapid that perforation occurs before a protective film forms.
This is usually seen with copper and its alloys because they efficient cathodes, even when filmed, and can stimulate anodic dissolution readily. This does not occur with stainless steels and other metals that form passive films.
Posted on: 7th April 2017
Alloys 400/K-500
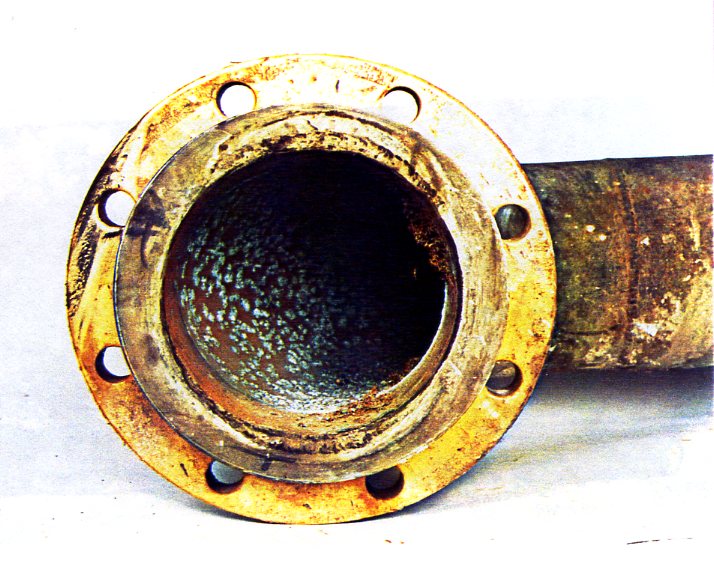
These materials (UNS N04400 and N05500) are nickel-copper alloys, with the K-500 grade being capable of having increased strength by precipitation hardening. The alloys were invented in the 1950's and have been widely used in seawater. Although they have good resistance to flowing seawater, they are susceptible to pitting and crevice corrosion, particularly in slow flowing natural seawater (as opposed to chlorinated seawater). The picture below shows a badly pitted alloy 400 seawater pipe from a Shell plant. Alloy K-500 has often been used for high strength bolting, but it can suffer crevice corrosion in seawater unless it is galvanically protected.
Alloys 400 and K-500 are not galvanically compatible with high alloy stainless steels and Ni-Cr-Mo alloys (e.g. alloy 625) in seawater, and will suffer accelerated atttack if coupled.
Because these alloys have a high nickel content, there are more cost-effective modern alloys that can meet many of the properties of alloys 400 and K-500. To replace them with copper alloys, it is coomon to select C70600 or C71500 instead of alloy 400, and C72420 as an alternative to K-500. Alloy 400 may also be replaced with cast or wrought superduplex stainless steel, while alloy K-500 may be replaced with cold worked superduplex or nickel alloys N07716 and N07725.
Posted on: 21st March 2017
Leaded Copper Alloys
These include leaded brasses, such as UNS C3800, manganese bronze and the leaded nickel silvers. The lead is usually present at 1 to 2 wt.% to improve machineability. The lead particles are fine and are distributed more or less uniformly throughout the matrix.
Sometimes these alloys are used for components that operate above the melting point of lead (~328°C). The author has seen these alloys used for items such as gas jets in a baker's oven and steam glands in a power station. At temperatures greater than 328°C the lead is very mobile and over a period of weeks to months it diffuses through the matrix and forms a small number of large lead particles. Where these are at the exposed surface, the liquid lead encourages fast diffusion and rapid corrosion can occur if air is present.
The solution is to use lead-free copper alloys or a chromium steel, such as the 7% or 9%Cr grades.
Posted on: 7th March 2017
Galvanized Steel
Galvanized steel piping is widely used to handle water, particularly fresh waters. It relies for its corrosion resistance on the formation of protective films of zinc and calcium carbonate. This means that medium and hard waters, with a total alkalinity>100mg/L (as CaCO3) and a positive Langelier Index will form protective scales. However, soft and particularly soft, acidic waters will not be able to form scale and they will corrode more rapidly. However, the low conductivity and low dissolved solids content of such waters means that the corrosion rate is usually low enough to be acceptable (<0.1mm/y).
In seawater galvanized steel offers no advantages over bare carbon steel. The reason for this is that, despite the total alkalinity of seawater exceeding 100mg/L, the high chloride content means that zinc corrosion products are soluble at normal seawater pH (8.2). Hence, the zinc layer dissolves rapidly and then the pipe corrodes just like carbon steel.
Posted on: 21st Feb 2017

